ISTANBUL, Jan. 28, 2026 (GLOBE NEWSWIRE) -- Memorial Şişli Hospital’s (part of Memorial Hospitals Group) Nuclear Medicine Department, Theranostics Unit has successfully completed the evaluation process under the European Association of Nuclear Medicine’s EANM–EARL framework and is now officially recognized as a Center of Excellence within the EARL Theranostics network.

Photo: Memorial Şişli Hospital via FL Comms.
The recognition is reflected in the EARL Programme 2025 “Theranostics Center — Qualified” badge, representing a significant quality milestone that confirms alignment with European standards and offers international patients and healthcare partners a clear assurance of standardized, high-quality care.
“Theranostics is transforming cancer care,” said Prof. Dr. Cüneyt Türkmen, Professor of Nuclear Medicine at Memorial Şişli Hospital. “By combining precise diagnosis with targeted radionuclide therapy, we can significantly improve survival and quality of life for patients with metastatic prostate cancer and neuroendocrine tumors—while minimizing side effects.”
Visual Source: Memorial Şişli Hospital via FL Comms.
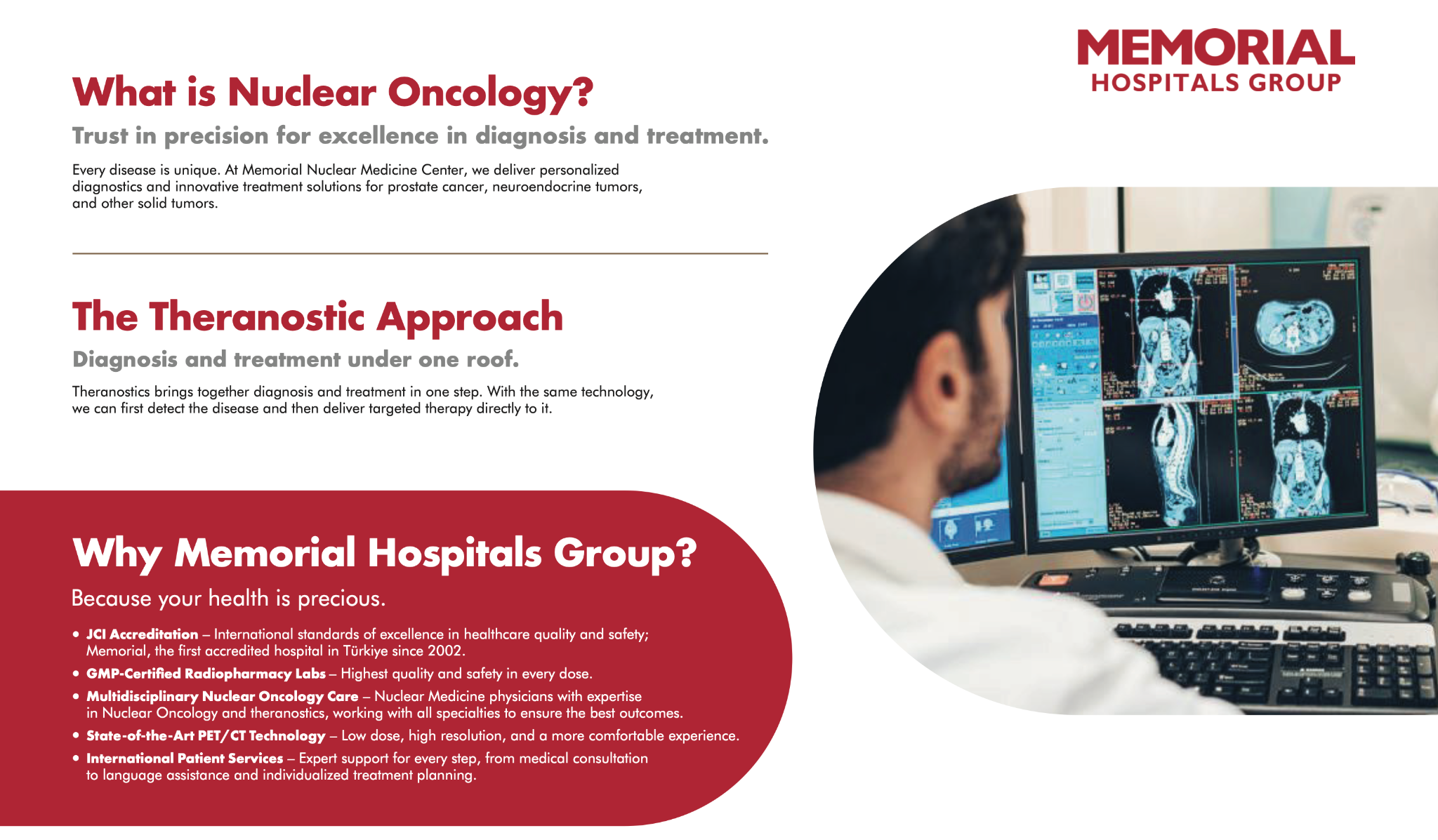
What theranostics means in clinical practice
Theranostics is a nuclear medicine approach that links diagnosis and treatment in a single, target-driven pathway, using the same biological target to first detect disease and then deliver therapy precisely to the affected tissue.
Memorial’s nuclear oncology capabilities
Memorial Şişli Hospital’s Nuclear Oncology and Theranostics Unit delivers advanced diagnostic and radioactive therapies for prostate cancer, neuroendocrine tumors, and selected solid tumors within the quality framework of the European Association of Nuclear Medicine (EANM).
For prostate cancer, targeted radionuclide therapies including Lutetium-177 (Lu-177) PSMA, Actinium-225 (Ac-225) PSMA, and Radium-223 (Ra-223) are offered, alongside theranostic treatments for neuroendocrine cancers such as Lutetium-177 (Lu-177) DOTATATE (PRRT) and Actinium-225 (Ac-225) DOTATATE. All procedures are performed in alignment with European standards for safety, quality, and clinical excellence.
Memorial Hospitals Group’s nuclear oncology program is built on personalized diagnostics and precision radionuclide therapies, supported by GMP-certified radiopharmacy laboratories, a multidisciplinary care model, and advanced PET/CT technology enabling low-dose, high-resolution imaging.
For international patients seeking standardized and reliable radioactive therapies, this recognition provides strong assurance that care at Memorial Şişli is delivered within a structured, audited, and internationally benchmarked framework. The EARL designation reflects both the scientific expertise of the medical team and the hospital’s commitment to precision-driven, patient-centered cancer care.
*Treatment decisions are made by the treating physician based on individual clinical evaluation.
About Memorial Hospitals Group
Memorial Hospitals Group states that it delivers “next-generation precision solutions in nuclear oncology from diagnosis to treatment,” with nuclear oncology centers across multiple sites, including Memorial Bahçelievler, Memorial Şişli, Memorial Göztepe, Memorial Ankara, Memorial Diyarbakır, and Medstar Antalya.
Memorial Şişli Hospital began admitting patients in February 2000 and, according to the hospital, received Joint Commission International (JCI) accreditation within two years of its founding. Memorial Şişli operates in a 53,000 m² indoor area with a capacity of 252 beds and serves international patients from 167 countries.
About EARL and EANM
EARL (EANM Research) is an EANM initiative focused on supporting quality and comparability in nuclear medicine and molecular imaging, including frameworks that promote standardized practice across centers.
EANM is a Europe-based nonprofit medical organization dedicated to nuclear medicine, providing a platform for scientific exchange and professional collaboration across the field.
Media Contact
Ugur Alkapar
Director of Global Media Relations
Email: ugur@flcommunications.co.uk
Photos accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/4b3f815f-83f7-4659-8a83-7c9923df97d1
https://www.globenewswire.com/NewsRoom/AttachmentNg/0bb2ab15-bf90-42b3-ac3d-ea3105a1df4e
https://www.globenewswire.com/NewsRoom/AttachmentNg/d4e12b64-55f9-49a9-8928-5c41b24785a5



