New York, USA, Sept. 22, 2025 (GLOBE NEWSWIRE) -- Thalassemia Treatment Market to Witness Significant Growth by 2034 Driven by Novel Drug Development | DelveInsight
The thalassemia market is primarily driven by regular blood transfusions and iron chelation therapies, which help manage anemia and prevent iron overload. Curative treatments, such as stem cell transplants, remain constrained due to limited donor availability. While there are currently no approved drugs for alpha thalassemia, several therapies are available for beta thalassemia, including CASGEVY, ZYNTEGLO, and REBLOZYL. Novel treatments like PYRUKYND, designed to reduce the need for transfusions, are anticipated to transform the long-term management of the disease.
DelveInsight’s Thalassemia Market Insights report includes a comprehensive understanding of current treatment practices, emerging thalassemia drugs, market share of individual therapies, and current and forecasted thalassemia market size from 2020 to 2034, segmented into leading markets (the US, EU4, UK, and Japan).
Thalassemia Market Summary
- The total thalassemia treatment market size is expected to grow positively by 2034 in the leading markets (the US, EU4, UK, and Japan).
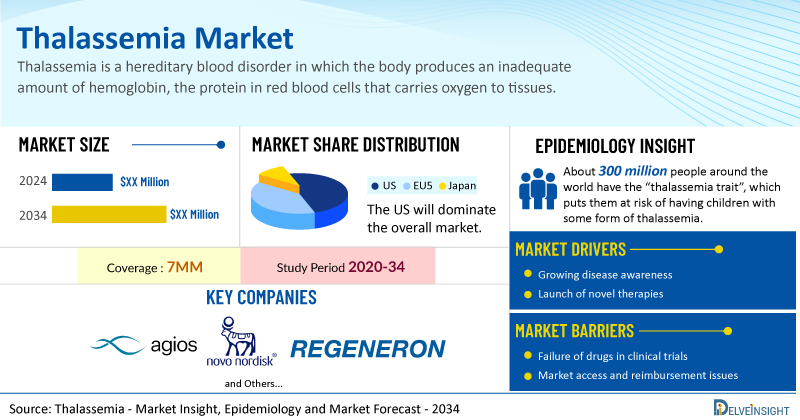
- The United States accounts for the largest market size of thalassemia, in comparison to EU4 (Germany, Italy, France, and Spain), the UK, and Japan.
- About 300 million people around the world have the “thalassemia trait”, which puts them at risk of having children with some form of thalassemia. More than 1 million people have non-transfusion-dependent thalassemia, while more than 100,000 people have transfusion-dependent thalassemia. In the US, there are at least 1,200 people with transfusion-dependent thalassemia.
- Key thalassemia companies, including Agios Pharmaceuticals, Novo Nordisk, Regeneron Pharmaceuticals, Bristol-Myers Squibb, and others, are actively working on innovative thalassemia drugs.
- Some of the key thalassemia therapies in clinical trials include PYRUKYND (mitapivat), Etavopivat (FT-4202), REGN7999, REBLOZYL, and others. These novel thalassemia therapies are anticipated to enter the Thalassemia market in the forecast period and are expected to change the market.
Discover which thalassemia medications are expected to grab the market share @ Thalassemia Market Report
Key Factors Driving the Growth of the Thalassemia Market
The growing diagnosed thalassemia patient pool drives the market
Improved surveillance, wider newborn/prenatal screening, and better reporting have increased the number of diagnosed thalassemia cases worldwide, enlarging the treated population and demand for therapies and diagnostics.
Emergence of a novel thalassemia drug class will propel the market
Pyruvate kinase activators like Agios Pharmaceuticals’ PYRUKYND are emerging as promising oral therapies for both transfusion-dependent and non-transfusion-dependent alpha- and beta-thalassemia. Regulatory approvals are underway in key markets, including the US and EU. On the other hand, Novo’s pyruvate kinase activator Etavopivat is also in a thalassemia clinical trial.
Better diagnostics & screening technologies will surge the market growth
Wider use of molecular diagnostics, NGS panels, carrier screening, and newborn screening programs improves early detection and stratification (who needs aggressive therapy versus conservative management). Earlier diagnosis both increases short-term utilization (confirmatory testing, genetic counseling) and long-term demand for therapies and monitoring.
Thalassemia Market Analysis
The management of alpha- and beta-thalassemia depends on the severity of the disease and may involve regular blood transfusions, iron chelation therapy to address iron overload, and curative approaches such as stem cell transplantation or gene therapy. Mild cases may not require any intervention, while newer targeted therapies are showing encouraging potential.
Currently, no therapies are approved for alpha-thalassemia. In contrast, several treatments are approved for beta-thalassemia, including CASGEVY (Vertex Pharmaceuticals), ZYNTEGLO (Bluebird Bio), and REBLOZYL (Bristol-Myers Squibb), among others. Gene therapy for β-thalassemia, including CASGEVY and ZYNTEGLO, employs genome editing techniques such as CRISPR-Cas9 or genetically modified stem cells to restore β-globin production. Although challenges remain, such as delivery efficiency and off-target effects, the use of zebrafish models and single-cell sequencing is improving both safety and precision.
Additionally, pyruvate kinase activators, like PYRUKYND, are emerging as promising oral treatments for both transfusion-dependent and non-transfusion-dependent forms of alpha- and beta-thalassemia.
Learn more about the thalassemia treatment options @ Thalassemia Treatment Market
Thalassemia Competitive Landscape
Key players, such as Agios Pharmaceuticals (PYRUKYND), Novo Nordisk (Etavopivat), Regeneron Pharmaceuticals (REGN7999), and others, are evaluating their lead candidates in different stages of clinical development. They aim to investigate their products for the treatment of thalassemia.
Agios Pharmaceuticals’ PYRUKYND is a pyruvate kinase activator approved in the U.S. for treating hemolytic anemia in adults with pyruvate kinase deficiency and in the European Union for adults with PK deficiency. The FDA recently extended the Prescription Drug User Fee Act (PDUFA) goal date for the supplemental New Drug Application (sNDA) by three months, moving the decision date to December 7, 2025. The sNDA seeks approval for use in adults with both non-transfusion-dependent (NTD) and transfusion-dependent (TD) alpha- or beta-thalassemia.
The extension follows an FDA information request, after which Agios submitted a proposed Risk Evaluation and Mitigation Strategy (REMS) to address the risk of hepatocellular injury noted in the original filing. Since the REMS counts as a major amendment, the FDA review period was extended. Importantly, the delay is not due to new or additional safety or efficacy data requested or provided.
Novo Nordisk’s Etavopivat is an oral small-molecule that activates the enzyme pyruvate kinase R (PKR). When PKR is activated, red blood cells generate more energy while 2,3-DPG levels decline. This dual effect enhances oxygen binding to defective hemoglobin, reducing the tendency of red blood cells to assume a sickle shape. As a result, red blood cell lifespan is extended, anemia is alleviated, and the frequency of vaso-occlusive crises (VOCs) decreases. The drug is currently under Phase III clinical evaluation for thalassemia and, according to the company’s pipeline, is also being investigated in Phase II for the same indication.
The anticipated launch of these emerging thalassemia therapies are poised to transform the thalassemia market landscape in the coming years. As these cutting-edge thalassemia therapies continue to mature and gain regulatory approval, they are expected to reshape the thalassemia market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
To know more about new treatment for thalassemia, visit @ Thalassemia Medication
Recent Developments in the Thalassemia Market
- In September 2025, Agios Pharmaceuticals, Inc. reported that the FDA had postponed the Prescription Drug User Fee Act (PDUFA) target action date for the supplemental New Drug Application (sNDA) of PYRUKYND (mitapivat)—an oral pyruvate kinase (PK) activator—by three months, moving it to December 7, 2025. The application seeks approval for use in adult patients with both non-transfusion-dependent (NTD) and transfusion-dependent (TD) alpha- or beta-thalassemia.
- In June 2025, Agios Pharmaceuticals presented new data on the company’s PK activators, mitapivat and tebapivat, which will be featured in oral and poster presentations during the 30th EHA Congress 2025.
What is Thalassemia?
Thalassemia is a hereditary blood disorder in which the body produces an inadequate amount of hemoglobin, the protein in red blood cells that carries oxygen to tissues. As a result, red blood cells are fewer in number and less effective, causing anemia, a condition characterized by fatigue, weakness, and other symptoms from reduced oxygen supply. The disorder is inherited from one or both parents and can range from mild cases with minimal symptoms to severe forms that require frequent blood transfusions. Thalassemia is more prevalent among individuals of Mediterranean, Middle Eastern, South Asian, and African descent. Depending on whether it affects the alpha or beta component of hemoglobin, it is classified as alpha or beta thalassemia.
Thalassemia Epidemiology Segmentation
The thalassemia epidemiology section provides insights into the historical and current thalassemia patient pool and forecasted trends for the leading markets. Worldwide, at least 300,000 children are born each year affected with a severe form of hemoglobinopathy. Furthermore, at least 20% of the global population carries at least a single gene deletion for alpha thalassemia.
The thalassemia market report proffers epidemiological analysis for the study period 2020–2034 in the leading markets (the US, EU4, UK, and Japan) segmented into:
- Total Diagnosed Prevalent Cases of Thalassemia
- Type-specific Diagnosed Prevalent Cases of Thalassemia
- Total Diagnosed Prevalent Cases of Thalassemia with Transfusion-dependency
- Total Treatable Cases of Thalassemia
Download the report to understand thalassemia disease management @ Thalassemia Treatment Options
| Thalassemia Market Report Metrics | Details |
| Study Period | 2020–2034 |
| Thalassemia Market Report Coverage | 7MM [The United States, the EU-4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan] |
| Thalassemia Epidemiology Segmentation | Total Diagnosed Prevalent Cases of Thalassemia, Type-specific Diagnosed Prevalent Cases of Thalassemia, Total Diagnosed Prevalent Cases of Thalassemia with Transfusion-dependency, and Total Treatable Cases of Thalassemia |
| Key Thalassemia Companies | Agios Pharmaceuticals, Novo Nordisk, Regeneron Pharmaceuticals, Vertex Pharmaceuticals, Bluebird Bio, Bristol-Myers Squibb, and others |
| Key Thalassemia Therapies | PYRUKYND (mitapivat), Etavopivat (FT-4202), REGN7999, CASGEVY, ZYNTEGLO, REBLOZYL, and others |
Scope of the Thalassemia Market Report
- Thalassemia Therapeutic Assessment: Thalassemia current marketed and emerging therapies
- Thalassemia Market Dynamics: Conjoint Analysis of Emerging Thalassemia Drugs
- Competitive Intelligence Analysis: SWOT analysis and Market entry strategies
- Thalassemia Market Unmet Needs, KOL’s views, Analyst’s views, Thalassemia Market Access and Reimbursement
Discover more about thalassemia drugs in development @ Thalassemia Clinical Trials
Table of Contents
| 1 | Thalassemia Market Key Insights |
| 2 | Thalassemia Market Report Introduction |
| 3 | Executive Summary of Thalassemia |
| 4 | Key Events of Thalassemia |
| 5 | Epidemiology and Market Methodology of Thalassemia |
| 6 | Thalassemia: Market Overview at a Glance |
| 6.1 | Total Market Share (%) Distribution of Thalassemia by Therapies in 2024 |
| 6.2 | Total Market Share (%) Distribution of Thalassemia by Therapies in 2034 |
| 7 | Disease Background and Overview: Thalassemia |
| 7.1 | Introduction |
| 7.2 | Thalassemia Risk Factors |
| 7.3 | Thalassemia Symptoms |
| 7.4 | Thalassemia Pathophysiology and Disease Pathways |
| 7.5 | Thalassemia Diagnostic Tests |
| 8 | Thalassemia Treatment and Guidelines |
| 8.1 | Current Treatment Landscape |
| 9 | Thalassemia Epidemiology and Patient Population |
| 9.1 | Key Findings |
| 9.2 | Assumptions and Rationale |
| 9.3 | Total Diagnosed Prevalent Cases of Thalassemia in the 7MM |
| 9.4 | The United States |
| 9.4.1 | Total Diagnosed Prevalent Cases of Thalassemia in the United States |
| 9.4.2 | Type-specific Diagnosed Prevalent Cases of Thalassemia in the United States |
| 9.4.3 | Total Diagnosed Prevalent Cases of Thalassemia With Transfusion-dependency in the United States |
| 9.4.4 | Total Treatable Cases of Thalassemia in the United States |
| 9.5 | EU4 and the UK |
| 9.6 | Japan |
| 10 | Patient Journey of Thalassemia |
| 11 | Marketed Drugs for Thalassemia |
| 11.1 | Key Competitors |
| 11.2 | CASGEVY (exagamglogene autotemcel): Vertex Pharmaceuticals |
| 11.2.1 | Product Description |
| 11.2.2 | Regulatory Milestones |
| 11.2.3 | Clinical Development |
| 11.2.4 | Safety and Efficacy |
| 11.2.5 | Analyst Views |
| 11.3 | REBLOZYL (luspatercept): Bristol-Myers Squibb |
| 12 | Emerging Therapies of Thalassemia |
| 12.1 | Key Competitors |
| 12.2 | PYRUKYND (mitapivat): Agios Pharmaceuticals |
| 12.2.1 | Product Description |
| 12.2.2 | Other Developmental Activities |
| 12.2.3 | Clinical Development |
| 12.2.4 | Safety and Efficacy |
| 12.2.5 | Analyst Views |
| 12.3 | Etavopivat (FT-4202): Novo Nordisk |
| List to be continued in the report… | |
| 13 | Thalassemia Market: Seven Major Market Size |
| 13.1 | Key Findings |
| 13.2 | Market Outlook of Thalassemia |
| 13.3 | Conjoint Analysis of Thalassemia |
| 13.4 | Key Market Forecast Assumptions of Thalassemia |
| 13.5 | Total Market Size of Thalassemia in the 7MM |
| 13.6 | United States Thalassemia Market Size |
| 13.6.1 | Total Market Size of Thalassemia in the United States |
| 13.6.2 | Total Market Size of Thalassemia by Therapies in the United States |
| 13.7 | EU4 and the UK Thalassemia Market Size |
| 13.8 | Japan Thalassemia Market Size |
| 14 | Unmet Needs of Thalassemia |
| 15 | SWOT Analysis of Thalassemia |
| 16 | KOL Views of Thalassemia |
| 17 | Market Access and Reimbursement of Thalassemia |
| 18 | Bibliography |
| 19 | Thalassemia Market Report Methodology |
Related Reports
Thalassemia Clinical Trial Analysis
Thalassemia Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key Thalassemia companies, including Novo Nordisk A/S, Kind Pharmaceuticals LLC, CorrectSequence Therapeutics Co., Ltd, Editas Medicine, San Rocco Therapeutics, Lantu Biopharma, Regeneron Pharmaceuticals, Kanglin Biotechnology (Hangzhou) Co., Ltd., Shanghai BDgene Co., Ltd., Bioray Laboratories, Celgene, Mabwell (Shanghai) Bioscience Co., Ltd., among others.
Alpha Thalassemia Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key alpha thalassemia companies, including Agios Pharmaceuticals, Forma Therapeutics, Silence Therapeutics, among others.
Alpha Thalassemia Clinical Trial Analysis
Alpha Thalassemia Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key alpha thalassemia companies, including Agios Pharmaceuticals, Inc., Bristol-Myers Squibb, among others.
Beta Thalassemia Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key beta thalassemia companies, including Novartis, Merck, Bristol Myers Squibb, Chiesi Farmaceutici S.p.A, Bluebird Bio, Agios Pharmaceuticals, Imara Inc., CRISPR Therapeutics, Vertex Pharmaceuticals, Vifor Pharma, Ionis Pharmaceuticals, Forma Therapeutics, DisperSol Technologies, SILENCE Therapeutics, among others.
Beta Thalassemia Clinical Trial Analysis
Beta Thalassemia Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key beta thalassemia companies, including CRISPR Therapeutics, CSL Vifor, Beam Therapeutics, EmeraMed, Fulcrum Therapeutics, Editas Medicine, EdiGene Inc., Silence Therapeutics, Phoenicia Biosciences, Shanghai BDgene, Disc Medicine, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter

Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com

